Elon Musk, the billionaire founder of Neuralink, announced on Monday via the social media platform X that the first human has successfully received an implant from the brain-chip startup on Sunday and is in good recovery. Last year, the U.S. Food and Drug Administration granted clearance to the company for its inaugural trial, testing the implant on humans.
Musk shared, "Initial results indicate promising neuron spike detection."
Neuralink's PRIME Study, focused on its wireless brain-computer interface, aims to evaluate the safety of the implant and surgical robot. The study is designed to assess the functionality of the interface, allowing individuals with quadriplegia to control devices using their thoughts, as outlined on the company's website.
Despite a Reuters request for additional information, Neuralink has not yet responded.
Earlier this month, Reuters reported that Neuralink faced fines for violating U.S. Department of Transportation (DOT) regulations related to the transportation of hazardous materials.
On Monday night, Musk shared on X:
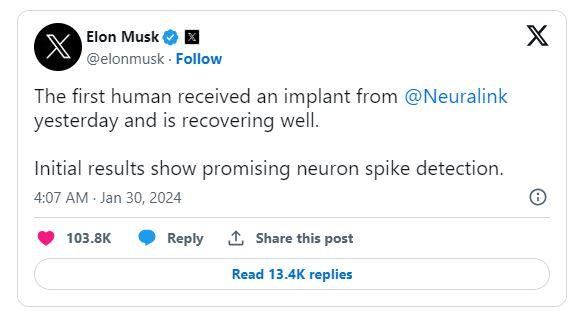
He explained that the device allows for the control of phones, computers, and nearly any device through mere thoughts. Musk expressed his vision, stating, 'Initial users will be those who have lost the use of their limbs. Imagine if Stephen Hawking could communicate faster than a speed typist or auctioneer. That is the goal.'
Neuralink is dedicated to implanting microchips into the brains of paralyzed individuals, enabling them to control their bodies through thoughts. In September, the company announced its plans to initiate a trial in humans to assess the safety of its implant.
Although the patient's details were not disclosed, Ashlee Vance, the author of a 2015 biography on Elon Musk, mentioned in a Bloomberg report that the ideal candidate for Neuralink's first human trial is 'an adult under age 40 whose four limbs are paralyzed.' Vance described the surgical process, involving a craniectomy and the robotic insertion of a chip into the brain region controlling hands, wrists, and forearms.
During the trial, a Neuralink-developed robot will place the implants' 'ultra-fine' threads that facilitate signal transmission in the participants' brains. Vance, who visited Neuralink's facilities multiple times, revealed Musk's drive to compete with other brain-computer startups like Synchron and Onward, which had already commenced human trials.
While Musk expressed frustration at the competition's progress, he urged Neuralink to accelerate its pace. Reuters reported a valuation of up to $5 billion for Neuralink based on private stock trades in June.
Despite controversies and skepticism from neuroscientists, Neuralink faced safety concerns, particularly with the FDA, in gaining approval for human trials. Issues related to the lithium battery, wire migration within the brain, and safe device extraction raised initial concerns. The FDA granted approval in May, but details on issue resolutions were not disclosed.
Musk's ambitious goals for Neuralink include using chip devices for conditions such as obesity, autism, depression, and schizophrenia, along with facilitating web browsing and telepathy. Experts caution that even if proven safe, it may take over a decade for Neuralink to secure commercial clearance.

















 English (United States) ·
English (United States) ·